by Jeremy E. Schreier
UGA Marine Sciences Ph.D. Student
It would not be field work without having to quickly adapt to rapidly changing scenarios – uncertainty always looming around the corner. A deteriorating weather forecast predicted rough seas not ideal for sampling, yet weather is not enough to completely stop scientific research. Taking advantage of the calm before the storm, the DolMICROBE07 team swiftly mobilized, moving the cruise a day earlier, interrupting final exams, and foregoing sampling at the station furthest from the coast. “As typical with all field oceanographic work, we are at the mercy of weather,” Marc Frischer wrote in a previous blog post . And that could not hold truer this time.
Residing in the surface ocean is a wide diversity of bacteria and phytoplankton, yet the control of a lab experiment allows scientists to test hypotheses that avoid the complexity of natural systems As a PhD candidate in the Department of Marine Sciences at the University of Georgia, Athens, my research focuses on marine microbial organisms, specifically bacteria and phytoplankton. I spend most of my time in a laboratory setting, allowing for a certain degree of control over the biological processes that occur within experiments conducted. For example, I mix salts together to make an artificial seawater to grow phytoplankton as an axenic monoculture (bacteria-free with only one organism). I add in a chosen bacterium, place in a growth chamber providing artificial sunlight at a specific temperature, and then observe the following interactions between.

Jeremy Schreier looking at a phytoplankton culture growing in artificial seawater in a laboratory growth chamber. For more about Jeremy, click the image.
Part of our training as scientists in the Marine Sciences PhD program at the University of Georgia requires us to participate in experiential learning, where we either conduct or assist in field research conducted by other scientists. As opposed to the laboratory, the field is dynamic and unpredictable, especially when you are on a mission for organisms such as the understudied gelatinous zooplankter, the doliolid. Recently, I had the opportunity to join the DolMICROBE research team aboard the RV Savannah.

DolMICROBE07 (RV SAV 21-08) science crew on the back deck of the RV Savannah around the mDPI. Front row from left: Marc Frischer and Savannah Geiger. Back row from the left: Jeremy Schreier, Anita Minniefield, Kyle Aaron, Laura Treible, Adam Greer, and Patrick Duffy.
Participating in fieldwork on a research vessel at sea requires thinking in more than three dimensions. Not only are there diverse organisms with complex life histories, many of which are millimeters to micrometers (1/1000th of a mm) in size, there are ocean currents and choppy surface waters (which cause seasickness), and abrupt changes in the physical and chemical properties through the water column (See our previous blog post). On top of all those uncontrollable forces is an aspect of time, one that only becomes more important when you are on the hunt for organisms that have bloom and bust periods such as doliolids. And if that was not enough, scientific instruments can fail, suddenly and unexpectedly.
To determine the properties of ocean water sampled, oceanographers rely on a CTD (stands for conductivity (i.e., salinity), temperature, and depth) on a rosette with Niskin bottles. The CTD rosette system on the RV Savannah measures 26 parameters that allow scientists to characterize the water column when it is deployed in the water. The system can also be used to collect and retrieve water in Niskin bottles at any depth allowing scientists to conduct additional measurements on water from specific depths. One of my jobs during this cruise was to operate the CTD console and ‘trigger’ the Niskin bottles to collect water that based on the sensor readings was identified for further study.
A key water property that is used in the DolMICROBE project to decide where to collect water is chlorophyll fluorescence. Chlorophyll is the green pigment that phytoplankton (and plants) use to perform photosynthesis. Because phytoplankton contain chlorophyll, chlorophyll fluorescence can be used to estimate how much phytoplankton are present, basically allowing scientists to “see” phytoplankton. According to Marc, phytoplankton along the continental shelf are often concentrated in deeper waters where nutrients are richer. When doliolids are present in the project study area, they are often most abundant in deeper water with higher concentrations of chlorophyll containing phytoplankton.
As operator of the CTD console, I was able to observe measurements in real time. While the chlorophyll sensor worked during our first CTD cast, at the second station fluorescence readings turned negative. Literally. The instrument read a negative number, indicating that it was not working properly. Research tech Morgan Hudgins was on the case! Alas, not even calls to customer service were able to remedy the solution.
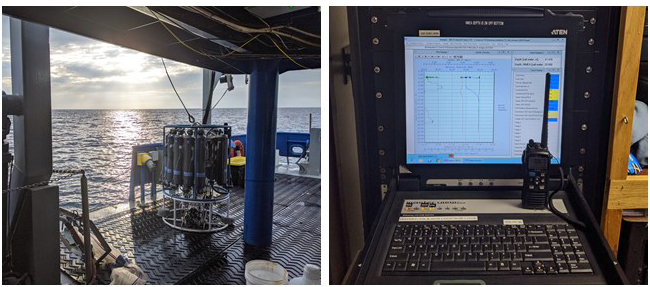
The CTD rosette system (left). Niskin bottles surround the scientific instruments in the center of the rosette. The CTD console in the ship (right) with water column properties being measured in real-time.
Quick to solve the problem, Adam Greer and his team of Laura Treible, Patrick Duffy, and Kyle Aaron were on board with their mDPI (mini Deep Focus Plankton Imager). A successful tow of this specialized instrument allowed us to see gelatinous zooplankton and estimate chlorophyll concentrations as, like the CTD, the mDPI has a chlorophyll fluorescence sensor onboard. As the mDPI was dragged through different depths of the water column in a ‘tow-yo’ (up and down) pattern, it provided us with a vision of relative phytoplankton abundance, allowing us to see the phytoplankton that we unable to without fluorescence measurements from the CTD.

The mDPI imaging system on the back deck of the RV Savannah (left). Top Right: Adam Greer, Laura Treible, and Patrick Duffy inspecting and testing the mDPI on deck. Middle Right: Adam Greer and Kyle Aaron deploying the mDPI. Bottom Right: Laura Treible and Patrick Duffy observing images streaming from the mDPI in real time.
After a night of successful sampling, we turned around and headed back towards shore to the shallow 25 m site, where we conducted routine sampling of the biological community. Although no dolioids were observed, the data collected will aid in better understanding the ecological function of microbial processes along the mid-continental shelf in the South Atlantic Bight. Additionally, successful deployment of the mDPI resulted in thousands of images that Adam Greer and Patrick Duffy will use as a window to resolve the vertical locations of gelatinous zooplankton, correlating them with other oceanographic properties measured.

Organisms observed during DolMICROBE-07. Top: (left) An amphipod parasite and (right) Slipper lobster larva (Phyllosoma) grabbing a chaetognath snack. Bottom: (left) A speedy Siphonophore and (right) a common salp (Salpa Fusiformis).
Upon the completion of sampling, we made our way back to the Skidaway Dock. As if right on cue, the storm began as the crewmates of the RV Savannah tied the boat to the dock. Our journey on DolMICROBE-07 ended with a downpour of rain, yet everyone was in high spirits and eager to analyze and processes the data collected. Field work is challenging and filled with complexity. While I spend most of my time in the laboratory, getting into the field always reinvigorates my passion for oceanography. Even when weather tries to intervene or equipment fails, observing science in action conducted in a natural laboratory such as the South Atlantic Bight broadened my perspective and knowledge of oceanographic research.

Jeremy Schreier, seasick but smiling.
Photos by M. Frischer, A. Greer, and J. Schreier