As Patrick reported in our last cruise log, in February we successfully collected and isolated several nurse and other life stages of Dolioletta gegenbauri. Marc and Tina have been diligently caring for this culture that will be used for experimental lab studies in the future. We currently have about 12 gonozoids in culture, with 4-5 appearing sexually mature. It is important that all team members know how to care for the culture, so I spent 3 days training with Marc this week learning the basics of taking care of these fragile organisms.
Doliolids are kept in glass jars filled with seawater in an incubation room set to a constant temperature (~18oC) and low light. The doliolid culture jars are secured on a slowly rotating plankton wheel that keeps the doliolids gently suspended in seawater (Fig. 1).
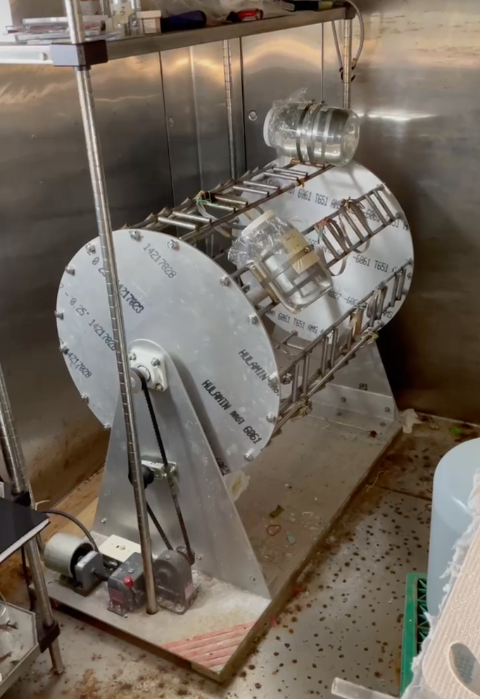
Figure 1: Plankton wheel with doliolid culture jars.
The routine daily maintenance of the culture can be broken down into two main parts: 1) visual assessment of the culture, and 2) feeding.
Assessment– When the jars are first removed from the wheel, the lids are gently removed and a small amount of water is taken out with a glass pipette so that water at the surface does not accidentally overflow a doliolid. Contents of each jar are visually examined to determine the relative amount of waste (fecal pellets) and the number of doliolids. Doliolids are counted, staged, and roughly measured with a graduated glass pipette. If the water looks ‘clean’ (low fecal pellets), no more water will be removed for cleaning. If there are a substantial number of large aggregates of fecal material, a glass pipette may be used with gentle suction to remove some of this material along with some of the water, however, doliolids don’t like it too clean. After all, a central hypothesis of the DolMICROBE project is that doliolids benefit nutritionally from the reconsumption of microbially re-worked detrital and fecal materials. Professor Paffenhöffer who originally developed this culturing strategy always kept “old” water in the jars to retain some of this material. Information is recorded in the culture notebook, noting doliolid abundance, stage, and general size, roughly how much water was removed for cleaning, and any other notes about the culture (Fig. 2).

Figure 2: Recording data in the culture notebook.
Feeding– To determine how much to feed each culture jar, we first need to determine the algal concentration or amount of food currently in each jar. Using a glass pipette, we remove ~100mL of water from each culture jar, being sure to collect water from the full depth of the jar (i.e., not just from the surface) and being careful to not remove any doliolids (Fig. 3). This is done for each culture jar, and noted in the culture notebook. These water samples are then covered with plastic wrap and placed into a small cooler to transport to the Coulter Counter.

Figure 3: Removing a sample of water from a culture jar with a glass pipette. This water sample will be used to determine current algal cell concentrations.
A Coulter Counter is “used to measure any particulate material that can be suspended in an electrolyte solution” (Fig. 4; https://www.beckman.com/resources/fundamentals/history-of-flow-cytometry/the-coulter-principle). We run the water samples through Coulter Counter to help determine the concentrations of algae that are currently in the culture jars to then calculate how much more algae we to add for feeding. When analyzing a sample, the Coulter Counter provides a distribution of different sizes of particles (Fig 4b).
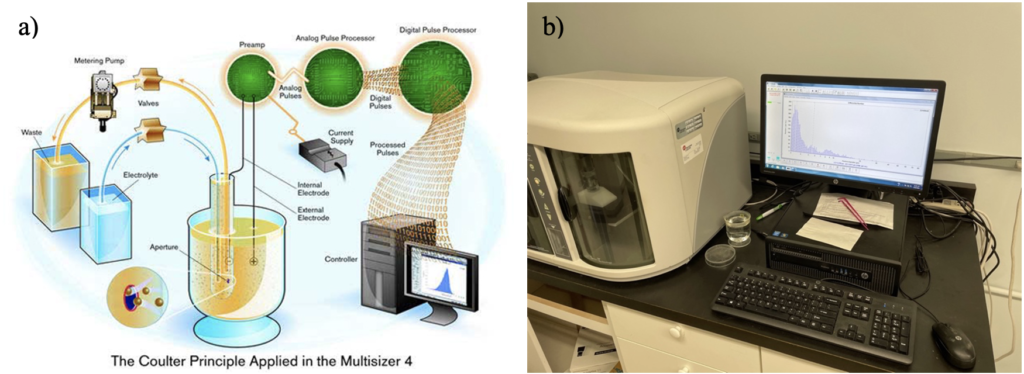
Figure 4: Coulter Counter. a) Schematic of a Coulter Counter (from https://www.beckman.com/). b) Running water sampled from doliolid culture jars on the SkIO Coulter Counter.
We feed the doliolids a mix of three algae species: Isochrysis galbana (Iso), Rhodomonas sp. (Rhodo), and Thalasia weissflogii (T. weiss). Each species has a different size range of cells, so we are able to estimate how much of the distribution of particles is contribution from each individual algal species. We record the cell counts in each species’ size range from the Coulter Counter and use a spreadsheet to calculate the amount of carbon (µg C/L) contribution from each species in each water sample. At the stage the culture was in during my training, we tried to keep ~ 80 µg C/L made up of 10 µgC/L of Iso, 30 µgC/L of Rhodo, and 40 µgC/L of T. weiss. These targets are rough estimates to make sure they have enough food to keep growing and (hopefully) reproduce, so we round up to the nearest mL when determining how much stock algae culture to add to each doliolid jar for feeding. We had to add mostly T. weiss and some Rhodo, but no Iso to feed the cultures this week.
After the doliolids are fed, it is time to get the jars back onto the wheel. Each jar is topped up to overflowing with filtered seawater, being careful not to overflow one of our precious doliolids. Once the jar is overflowing with water, a sheet of plastic wrap is gently placed over the surface of the water (Fig. 5) and the lid is secured on top. This ensures that there is no air in the header of the jar, so that everything in the water is in gentle suspension as the jars rotate around on the wheel.

Figure 5: Placing plastic wrap over the water surface before securing the lid on a doliolid culture jar.
After the jars are secured onto the wheel with hose clamps (Fig. 6) and the wheel is turned back on to rotate slowly, we say ‘good luck!’ to the doliolids and hope they continue to grow and reproduce!

Figure 6: Tightening the hose clamps to secure a doliolid culture jar on the plankton wheel.
For more information about culturing doliolids please read our manuscript in the Journal of Visualized Experiments (citation below) and watch a training video here (https://outlookuga-my.sharepoint.com/:v:/g/personal/frischer_uga_edu/EfecVHlVZgFGuU1de-ut5yQBvUwhcmxTP0QYwP1Ha8OQzQ?e=J41Tm6)
Walters, T.L., D.M. Gibson, M.E. Frischer (2019). Cultivation of the marine pelagic tunicate Dolioletta gegenbauri (Uljanin 1884) for experimental studies. Journal of Visualized Experiments 150: e59832, doi:10.3791/59832.